1. RISK CLASSES AND CONFORMITY ASSESSMENT PROCEDURES
CMI is the designated notified body under the MDR for conformity assessment of medical devices of risk classes:
- Is - medical devices placed on the market in sterile condition
- Im – medical devices with measuring function
- Ir – reusable surgical instruments
- IIa
- IIb
for the following conformity assessment procedures:
- Annex IX, Chapter I and III MDR – Conformity assessment based on a quality management system
- Annex IX, Chapter II MDR - Conformity assessment based on assessment of technical documentation
- Annex XI, Part A MDR - Conformity assessment based on product conformity verification – production quality assurance (only for classes Is, Im, Ir, and IIa)
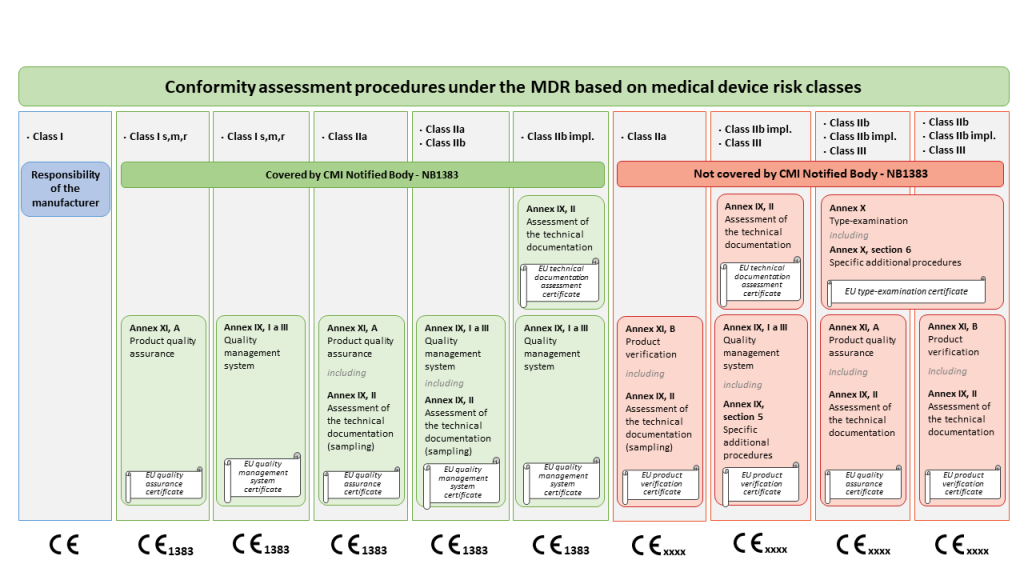
The MDR risk-based conformity assessment procedures that the Czech Metrology Institute can provide, can also be found in the graphic below.
2. MDR CODES
CMI has a range of 20 basic MDR codes and 18 horizontal MDR codes. Specifically, the following:
2.1 Basic codes
Active non-implantable devices for imaging, monitoring, and / or diagnosis
- MDA 0201 - Active non-implantable imaging devices utilising ionizing radiation
- MDA 0202 - Active non-implantable imaging devices utilising non-ionizing radiation
- MDA 0203 - Active non-implantable devices for monitoring of vital physiological parameters
- MDA 0204 - Other active non-implantable devices for monitoring and / or diagnosis
Active non-implantable therapeutic devices and general active non-implantable devices
- MDA 0301 - Active non-implantable devices utilising ionizing radiation
- MDA 0302 - Active non-implantable devices utilising non-ionizing radiation
- MDA 0305 – Active non-implantable devices for stimulation or inhibition
- MDA 0307 – Active non-implantable respiratory devices
- MDA 0312 – Other active non-implantable surgical devices
- MDA 0315 – Software
- MDA 0316 – Medical gas supply systems and parts thereof
- MDA 0317 – Active non-implantable devices for cleaning, disinfection and sterilisation
Non-active implants and long term surgically invasive devices
- MDN 1102 – Non-active osteo- and orthopaedic implants
- MDN 1103 – Non-active dental implants and dental materials
Non-active non-implantable devices
- MDN 1205 – Non-active non-implantable orthopaedic and rehabilitation devices
- MDN 1207 – Non-active non-implantable diagnostic devices
- MDN 1208 – Non-active non-implantable instruments
- MDN 1209 – Non-active non-implantable dental materials
- MDN 1211 – Non-active non-implantable devices for disinfecting, cleaning and rinsing
- MDN 1214 – General non-active non-implantable devices used in health care and other nonactive non-implantable devices
We are currently in process of obtaining designation for the following codes:
MDA 0303, 0304, 0306, 0313, 0318
MDN 1201, 1202, 1203, 1204, 1213
2.2 Horizontal codes
Devices with specific characteristics
- MDS 1004 – Devices which are also machinery as defined in point (a) of the second paragraph of Article 2 of Directive 2006/42/EC of the European Parliament and of the Council
- MDS 1005 – Devices in sterile condition
- MDS 1006 – Reusable surgical instruments
- MDS 1007 – Devices incorporating or consisting of nanomaterial
- MDS 1009 – Devices incorporating software / utilising software / controlled by software, including devices intended for controlling, monitoring or directly influencing the performance of active or active implantable devices
- MDS 1010 – Devices with a measuring function
- MDS 1011 - Devices in systems or procedure packs
- MDS 1012 – Products without an intended medical purpose listed in Annex XVI to Regulation (EU) 2017/745
Devices for which specific technologies or processes are used
- MDT 2001 – Devices manufactured using metal processing
- MDT 2002 – Devices manufactured using plastic processing
- MDT 2003 – Devices manufactured using non-metal mineral processing (e.g. glass, ceramics)
- MDT 2004 – Devices manufactured using non-metal non-mineral processing (e.g. textiles, rubber, leather, paper)
- MDT 2005 – Devices manufactured using biotechnology
- MDT 2006 – Devices manufactured using chemical processing
- MDT 2008 – Devices manufactured in clean rooms and associated controlled environments
- MDT 2010 – Devices manufactured using electronic components including communication devices
- MDT 2011 – Devices which require packaging, including labelling
- MDT 2012 – Devices which require installation, refurbishment
3. LIMITATIONS OF THE CODES LISTED
CMI has the following limitations for the above codes:
- All MDA and MDN codes are limited to no class III devices and, using the Annex XI (A) conformity assessment procedure, no class IIb devices
- MDN 1214 is limited to incontinence devices only
- MDS 1005 is limited to the following sterilisation methods:
- Aseptic processing
- Sterilization with ethylene oxide (ETO)
- Sterilization by moist heat
- Dry heat sterilization
- Radiation - gamma only
- MDS 1012 is limited to devices emitting high intensity electromagnetic radiation (e.g., infrared, visible, or ultraviolet light) intended for use on the human body, including coherent and incoherent sources, monochromatic or broad spectrum, such as lasers and intense pulsed light devices, for skin resurfacing, tattooing or hair removal, or other skin treatments.
